ɀ ɍ Ɋ ɇ Ⱥ Ʌ ɋ Ɍ Ɋ ɍ... Ɇɚɣ – ɢɸɧɶ ɋ. 590 – 593 2006. Ɍɨɦ 47, ʋ 3
реклама

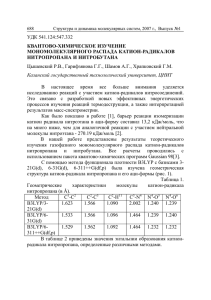
ɀɍɊɇȺɅ ɋɌɊɍɄɌɍɊɇɈɃ ɏɂɆɂɂ 2006. Ɍɨɦ 47, ʋ 3 Ɇɚɣ – ɢɸɧɶ ɋ. 590 – 593 ɄɊȺɌɄɂȿ ɋɈɈȻɓȿɇɂə ɍȾɄ 548.736 ɄɊɂɋɌȺɅɅɂɑȿɋɄȺə ɋɌɊɍɄɌɍɊȺ ȾɂȽɂȾɊȺɌȺ ȾɂȽɂȾɊɈɗɌɂɅȿɇȾɂȺɆɂɇɌȿɌɊȺȺɐȿɌȺɌȺ(2–) ɄȺɅɖɐɂə Ca(H2Edta)·2H2O 2006 Ɇ . ɐ ɚ ɛ ɟ ɥ ɶ 1 * , Ⱥ . Ʌ . ɉ ɨ ɡ ɧ ɹ ɤ 2 , ȼ . ɂ . ɉ ɚ ɜ ɥ ɨ ɜ ɫ ɤ ɢ ɣ 2 1 ɂɧɫɬɢɬɭɬ ɧɟɨɪɝɚɧɢɱɟɫɤɨɣ ɯɢɦɢɢ ɭɧɢɜɟɪɫɢɬɟɬɚ Ɋɟɝɟɧɫɛɭɪɝɚ, Ƚɟɪɦɚɧɢɹ ɂɧɫɬɢɬɭɬ ɦɨɥɟɤɭɥɹɪɧɨɣ ɢ ɚɬɨɦɧɨɣ ɮɢɡɢɤɢ ɇȺɇ Ȼɟɥɚɪɭɫɢ, Ɇɢɧɫɤ 2 ɋɬɚɬɶɹ ɩɨɫɬɭɩɢɥɚ 11 ɨɤɬɹɛɪɹ 2005 ɝ. Ʉɪɢɫɬɚɥɥɵ Ca(H2Edta)2H2O (ɪɨɦɛɢɱɟɫɤɚɹ ɫɢɧɝɨɧɢɹ, a = 8,5919(7), b = 17,807(2), c = 18,941(2) Å; Z = 8, ɩɪɨɫɬɪɚɧɫɬɜɟɧɧɚɹ ɝɪɭɩɩɚ Pbca) ɜɵɩɚɞɚɸɬ ɢɡ ɪɚɫɬɜɨɪɨɜ Na2H2Edta2H2O ɢ CaCl2. Ⱥɬɨɦ Ca ɨɤɪɭɠɟɧ ɩɨ ɜɟɪɲɢɧɚɦ ɢɫɤɚɠɟɧɧɨɝɨ ɨɤɬɚɷɞɪɚ ɚɬɨɦɚɦɢ ɤɢɫɥɨɪɨɞɚ ɦɨɥɟɤɭɥɵ ɜɨɞɵ ɢ ɤɚɪɛɨɤɫɢɥɶɧɵɯ ɝɪɭɩɩ ɩɹɬɢ ɫɨɫɟɞɧɢɯ ɚɧɢɨɧɨɜ H2Edta2– ɫ ɩɪɨɬɨɧɢɪɨɜɚɧɧɵɦɢ ɚɬɨɦɚɦɢ ɚɡɨɬɚ. ȼ ɪɟɡɭɥɶɬɚɬɟ ɨɛɪɚɡɭɟɬɫɹ ɬɪɟɯɦɟɪɧɵɣ ɤɚɪɤɚɫ [Ca(H2O)H2Edta)], ɜ ɤɨɬɨɪɵɣ ɜɫɬɪɨɟɧɵ ɦɨɥɟɤɭɥɵ ɤɪɢɫɬɚɥɥɢɡɚɰɢɨɧɧɨɣ ɜɨɞɵ. Ʉ ɥ ɸ ɱ ɟ ɜ ɵ ɟ ɫ ɥ ɨ ɜ ɚ : ɤɪɢɫɬɚɥɥɢɱɟɫɤɚɹ ɫɬɪɭɤɬɭɪɚ, ɞɢɝɢɞɪɨɷɬɢɥɟɧɞɢɚɦɢɧɬɟɬɪɚɚɰɟɬɚɬ ɤɚɥɶɰɢɹ. ȼ ɫɨɟɞɢɧɟɧɢɹɯ ɷɬɢɥɟɧɞɢɚɦɢɧɬɟɬɪɚɭɤɫɭɫɧɨɣ ɤɢɫɥɨɬɵ H4Edta ɫ ɧɟɤɨɬɨɪɵɦɢ ɦɟɬɚɥɥɚɦɢ ɦɨɝɭɬ ɩɪɢɫɭɬɫɬɜɨɜɚɬɶ ɢɨɧɵ H2Edta2–, ɜ ɤɨɬɨɪɵɯ ɚɬɨɦɵ ɚɡɨɬɚ ɩɪɨɬɨɧɢɪɨɜɚɧɵ. ɇɚ ɷɬɨ ɭɤɚɡɵɜɚɸɬ, ɜ ɱɚɫɬɧɨɫɬɢ, ɪɟɧɬɝɟɧɨɫɬɪɭɤɬɭɪɧɵɟ ɢɫɫɥɟɞɨɜɚɧɢɹ ɪɹɞɚ ɫɨɟɞɢɧɟɧɢɣ: Ɇ2(H2Edta)2H2O, ɝɞɟ Ɇ = Na (IIa), K (IIb), Rb (IIc) [ 1 — 3 ], ɚ ɬɚɤɠɟ Mg(H2Edta)6H2O (III) [ 4 ]. Ɇɵ ɩɪɢɝɨɬɨɜɢɥɢ ɤɪɢɫɬɚɥɥɵ ɧɨɜɨɝɨ ɫɨɟɞɢɧɟɧɢɹ ɷɬɨɝɨ ɪɹɞɚ, ɚ ɢɦɟɧɧɨ Ca(H2Edta)2H2O (I), ɢ ɜɵɩɨɥɧɢɥɢ ɢɯ ɪɟɧɬɝɟɧɨɫɬɪɭɤɬɭɪɧɵɣ ɚɧɚɥɢɡ. ɗɤɫɩɟɪɢɦɟɧɬɚɥɶɧɚɹ ɱɚɫɬɶ. Ʉɪɢɫɬɚɥɥɵ I ɨɛɪɚɡɭɸɬɫɹ ɩɪɢ ɦɟɞɥɟɧɧɨɦ ɨɯɥɚɠɞɟɧɢɢ ɝɨɪɹɱɟɝɨ (90 qɋ) ɪɚɫɬɜɨɪɚ Na2H2Edta2H2O ɢ CaCl2, ɜɡɹɬɵɯ ɜ ɫɨɨɬɧɨɲɟɧɢɢ 2:1. ɉɚɪɚɦɟɬɪɵ ɷɥɟɦɟɧɬɚɪɧɨɣ ɹɱɟɣɤɢ ɢ ɬɪɟɯɦɟɪɧɵɣ ɧɚɛɨɪ ɢɧɬɟɧɫɢɜɧɨɫɬɟɣ ɨɬɪɚɠɟɧɢɣ ɩɨɥɭɱɟɧɵ ɞɥɹ ɦɨɧɨɤɪɢɫɬɚɥɥɚ ɪɚɡɦɟɪɨɦ 0,66u0,52u0,50 ɦɦ ɧɚ ɞɢɮɪɚɤɬɨɦɟɬɪɟ STOE-IPDS (ɢɡɥɭɱɟɧɢɟ MoKD, ɝɪɚɮɢɬɨɜɵɣ ɦɨɧɨɯɪɨɦɚɬɨɪ) ɩɪɢ ɬɟɦɩɟɪɚɬɭɪɟ 173(1) K (ɨɯɥɚɠɞɚɸɳɚɹ ɩɪɢɫɬɚɜɤɚ Oxford Cryosystems). Ʉɪɢɫɬɚɥɥɵ C10H18CaN2O10 (M = 366,64) ɪɨɦɛɢɱɟɫɤɢɟ: a = 8,5919(7), b = 17,807(2), c = 18,941(2) Å; V = 2897,4(4) Å3, Z = 8, Uɜɵɱ = 1,679 ɝ/ɫɦ3, PMo = 0,492 ɦɦ–1, F(000) = 1536, ɩɪɨɫɬɪɚɧɫɬɜɟɧɧɚɹ ɝɪɭɩɩɚ Pbca. ȼɫɟɝɨ ɡɚɪɟɝɢɫɬɪɢɪɨɜɚɧɨ 37025 ɪɟɮɥɟɤɫɨɜ (T = 2,15—26,91º, ɢɧɬɟɪɜɚɥ ɢɧɞɟɤɫɨɜ –10 d h d 10, –22 d k d 22, –23 d l d 23), ɢɡ ɧɢɯ 3120 ɧɟɡɚɜɢɫɢɦɵɯ ɪɟɮɥɟɤɫɨɜ (Rint = 0,0344). ɉɨɩɪɚɜɤɚ ɧɚ ɩɨɝɥɨɳɟɧɢɟ ɭɱɢɬɵɜɚɥɚɫɶ ɱɢɫɥɟɧɧɵɦ ɦɟɬɨɞɨɦ ɩɨ ɮɨɪɦɟ ɤɪɢɫɬɚɥɥɚ (Tmin = 0,7470, Tmax = 0,8116). ɋɬɪɭɤɬɭɪɚ ɪɚɫɲɢɮɪɨɜɚɧɚ ɩɪɹɦɵɦ ɦɟɬɨɞɨɦ (SIR-97 [ 5 ] ) ɢ ɭɬɨɱɧɟɧɚ ɩɨɥɧɨɦɚɬɪɢɱɧɵɦ ɚɧɢɡɨɬɪɨɩɧɵɦ ɦɟɬɨɞɨɦ ɧɚɢɦɟɧɶɲɢɯ ɤɜɚɞɪɚɬɨɜ (SHELX-97 [ 6 ] ). Ⱥɬɨɦɵ H ɦɟɬɢɥɟɧɨɜɵɯ ɝɪɭɩɩ ɩɨɦɟɳɟɧɵ ɜ ɫɨɨɬɜɟɬɫɬɜɢɢ ɫ ɝɟɨɦɟɬɪɢɟɣ ɢ ɭɬɨɱɧɟɧɵ ɩɨ ɦɨɞɟɥɢ sɧɚɟɡɞɧɢɤɚs, ɨɫɬɚɥɶɧɵɟ — ɢɡ ɪɚɡɧɨɫɬɧɵɯ ɫɢɧɬɟɡɨɜ [ 6 ]. Ɉɤɨɧɱɚɬɟɥɶɧɵɟ ɩɚɪɚɦɟɬɪɵ ɭɬɨɱɧɟɧɢɹ: R1 = 0,0270, wR2 = 0,0715 ɞɥɹ 2884 ɨɬɪɚɠɟɧɢɣ ɫ I > 2V(I ), R1 = 0,0293, wR2 = 0,0729 ɞɥɹ ɜɫɟɯ ɨɬɪɚɠɟɧɢɣ (262 ɭɬɨɱɧɹɟɦɵɯ ɩɚɪɚɦɟɬɪɚ), GOOF = 1,049. Ɇɚɤɫɢɦɚɥɶɧɚɹ ɢ ɦɢɧɢɦɚɥɶɧɚɹ ɨɫɬɚɬɨɱɧɚɹ ɷɥɟɤɬɪɨɧɧɵɟ ɩɥɨɬɧɨɫɬɢ ɫɨɫɬɚɜɥɹɸɬ 0,334 ɢ –0,282 e·Å–3 ɫɨɨɬɜɟɬɫɬɜɟɧɧɨ. Ʉɨɨɪɞɢɧɚɬɵ ɢ ɬɟɩɥɨɜɵɟ ɩɚɪɚɦɟɬɪɵ ɚɬɨɦɨɜ ɩɪɢɜɟɞɟɧɵ ɜ ɬɚɛɥ. 1, ɞɥɢɧɵ ɫɜɹɡɟɣ ɢ ɜɚɥɟɧɬɧɵɟ ɭɝɥɵ — ɜ ɬɚɛɥ. 2. * E-mail: manfred.zabel@chemie.uni-regensburg.de 591 ɄɊȺɌɄɂȿ ɋɈɈȻɓȿɇɂə Ɍɚɛɥɢɰɚ 1 4 2 3 Ʉɨɨɪɞɢɧɚɬɵ ɚɬɨɦɨɜ (u10 ) ɢ ɬɟɩɥɨɜɵɟ ɩɚɪɚɦɟɬɪɵ (Å u10 ) Ⱥɬɨɦ x y z Ueq* Ⱥɬɨɦ x y z Ueq* Ca(1) O(1) O(2) O(3) O(4) O(5) O(6) O(7) O(8) O(1w) N(1) N(2) C(1) 7760(1) 9403(1) 8652(1) 9991(1) 10698(1) 9932(1) 9289(1) 10284(1) 8825(1) 6735(2) 7943(1) 7938(1) 7752(2) 835(1) 2157(1) 3069(1) 4214(1) 3427(1) 1729(1) 1034(1) 29(1) –539(1) –292(1) 2760(1) 1020(1) 3139(1) 6430(1) 2774(1) 2027(1) 4869(1) 4004(1) 4489(1) 5432(1) 3216(1) 2395(1) 5993(1) 3913(1) 3601(1) 3208(1) 15(1) 23(1) 21(1) 33(1) 23(1) 22(1) 24(1) 20(1) 26(1) 43(1) 15(1) 16(1) 18(1) C(2) C(3) C(4) C(5) C(6) C(7) C(8) C(9) C(10) O(2w) H(1N) H(2N) 8693(1) 8145(2) 9750(1) 6658(1) 6553(1) 8225(2) 9231(1) 7651(2) 9051(1) 7273(1) 8785(19) 8788(19) 2748(1) 3320(1) 3691(1) 2216(1) 1552(1) 681(1) 1199(1) 441(1) –66(1) –1786(1) 2516(9) 1287(9) 2628(1) 4495(1) 4446(1) 4090(1) 3589(1) 4322(1) 4787(1) 3037(1) 2872(1) 6240(1) 3908(8) 3467(8) 18(1) 18(1) 18(1) 18(1) 17(1) 18(1) 18(1) 19(1) 17(1) 41(1) 18 19 * Ueq ɨɩɪɟɞɟɥɟɧɵ ɤɚɤ ɨɞɧɚ ɬɪɟɬɶ ɫɥɟɞɚ ɨɪɬɨɝɨɧɚɥɢɡɨɜɚɧɧɨɝɨ ɬɟɧɡɨɪɚ Uij. Ɍɚɛɥɢɰɚ 2 Ⱦɥɢɧɵ ɫɜɹɡɟɣ d, Å ɋɜɹɡɶ d ɋɜɹɡɶ d ɋɜɹɡɶ d Ca(1)—O(6) Ca(1)—O(1w) Ca(1)—O(8)#1 Ca(1)—O(4)#2 Ca(1)—O(7)#3 Ca(1)—O(2)#4 O(1)—C(2) O(2)—C(2) O(3)—C(4) 2,3298(9) 2,3442(12) 2,3391(10) 2,3546(10) 2,3748(9) 2,3811(9) 1,2469(15) 1,2737(14) 1,2443(16) O(4)—C(4) O(5)—C(8) O(6)—C(8) O(7)—C(10) O(8)—C(10) N(1)—C(1) N(1)—C(5) N(1)—C(3) N(2)—C(6) 1,2596(15) 1,2529(15) 1,2577(14) 1,2548(15) 1,2507(14) 1,5062(15) 1,5067(16) 1,4954(16) 1,5207(16) N(2)—C(7) N(2)—C(9) N(1)—H(1N) N(2)—H(2N) C(1)—C(2) C(3)—C(4) C(5)—C(6) C(7)—C(8) C(9)—C(10) 1,5123(15) 1,5057(16) 0,845(16) 0,908(16) 1,5321(17) 1,5320(18) 1,5195(17) 1,5406(17) 1,5358(17) ɉ ɪ ɢ ɦ ɟ ɱ ɚ ɧ ɢ ɟ. Ɉɩɟɪɚɰɢɢ ɫɢɦɦɟɬɪɢɢ: #1 3/2–x, –y, 1/2+z; #2 x–1/2, 1/2–y, 1—z; #3 2–x, –y, 1–z; #4 x, 1/2–y, 1/2+z. Ɇɨɥɟɤɭɥɹɪɧɚɹ ɝɪɚɮɢɤɚ ɜɵɩɨɥɧɟɧɚ ɫ ɩɨɦɨɳɶɸ ɩɪɨɝɪɚɦɦɵ PLATON [ 7 ]. Ɋɟɡɭɥɶɬɚɬɵ ɢ ɢɯ ɨɛɫɭɠɞɟɧɢɟ. Ʉɪɢɫɬɚɥɥɵ I ɫɨɫɬɨɹɬ ɢɡ ɢɨɧɨɜ ɤɚɥɶɰɢɹ ɢ H2Edta2–, ɚ ɬɚɤɠɟ ɦɨɥɟɤɭɥ ɜɨɞɵ. ȼ ɤɨɨɪɞɢɧɚɰɢɨɧɧɨɣ ɫɮɟɪɟ ɋɚ (ɢɫɤɚɠɟɧɧɵɣ ɨɤɬɚɷɞɪ) ɧɚɯɨɞɢɬɫɹ ɚɬɨɦ O(1w) ɦɨɥɟɤɭɥɵ ɜɨɞɵ ɢ ɩɹɬɶ ɚɬɨɦɨɜ ɤɢɫɥɨɪɨɞɚ ɤɚɪɛɨɤɫɢɥɶɧɵɯ ɝɪɭɩɩ ɩɹɬɢ ɫɨɫɟɞɧɢɯ ɢɨɧɨɜ H2Edta2– (ɪɢɫ. 1, ɬɚɛɥ. 2). ȼ ɫɜɨɸ ɨɱɟɪɟɞɶ, ɜ ɫɨɝɥɚɫɢɢ ɫɨ ɫɬɟɯɢɨɦɟɬɪɢɟɣ ɫɨɟɞɢɧɟɧɢɹ, ɤɚɠɞɵɣ ɚɧɢɨɧ H2Edta2– ɫɜɹɡɚɧ ɫ ɩɹɬɶɸ ɚɬɨɦɚɦɢ ɋɚ, ɜ ɪɟɡɭɥɶɬɚɬɟ ɱɟɝɨ ɢɡ ɷɬɢɯ ɫɨɫɬɚɜɥɹɸɳɢɯ ɨɛɪɚɡɭɟɬɫɹ ɬɪɟɯɦɟɪɧɚɹ ɫɟɬɱɚɬɚɹ ɫɬɪɭɤɬɭɪɚ (ɪɢɫ. 2). Ɏɨɪɦɢɪɨɜɚɧɢɟ ɟɟ ɦɨɠɧɨ ɩɪɟɞɫɬɚɜɢɬɶ ɫɥɟɞɭɸɳɢɦ ɨɛɪɚɡɨɦ. Ⱦɜɚ ɚɬɨɦɚ ɋɚ ɢ ɞɜɚ ɚɧɢɨɧɚ H2Edta2–, ɫɨɨɬɧɨɫɹɳɢɟɫɹ ɫ ɰɟɧɬɪɨɦ ɫɢɦɦɟɬɪɢɢ, ɨɛɴɟɞɢɧɹɸɬɫɹ ɜ ɞɢɦɟɪ. ȼ ɨɫɧɨɜɟ ɞɢɦɟɪɚ 16-ɱɥɟɧɧɵɣ ɰɢɤɥ (ɫɦ. ɪɢɫ. 1), ɩɨɫɤɨɥɶɤɭ ɞɜɚ ɚɬɨɦɚ ɋɚ ɜ ɧɟɦ ɫɜɹɡɚɧɵ ɦɟɠɞɭ ɫɨɛɨɣ ɚɰɟɬɚɬɧɵɦɢ ɝɪɭɩɩɚɦɢ, ɩɪɢɧɚɞɥɟɠɚɳɢɦɢ ɨɞɧɨɦɭ ɚɬɨɦɭ ɚɡɨɬɚ ɚɧɢɨɧɚ H2Edta2–, ɫ ɭɱɚɫɬɢɟɦ ɚɬɨɦɨɜ Ɉ(6) ɢ Ɉ(7). ȿɫɥɢ ɩɪɢɧɹɬɶ ɜɨ ɜɧɢɦɚɧɢɟ ɬɨɥɶɤɨ ɚɬɨɦɵ Ɉ(7) ɢ Ɉ(8), ɩɪɢɧɚɞɥɟɠɚɳɢɟ ɨɞɧɨɣ ɤɚɪɛɨɤɫɢɥɶɧɨɣ ɝɪɭɩɩɟ, ɬɨ ɚɬɨɦɵ ɋɚ ɨɤɚɠɭɬɫɹ ɜ ɰɟɩɨɱɤɟ —ɋɚ—Ɉ(7)—ɋ—Ɉ(8)—ɋɚ— Ɉ(7)—ɋ—Ɉ(8)—ɋɚ— ɜɞɨɥɶ ɨɫɢ x, ɜ ɤɨɬɨɪɨɣ ɤɚɠɞɵɣ ɢɡ ɚɬɨɦɨɜ ɭɝɥɟɪɨɞɚ ɩɪɢɧɚɞɥɟɠɢɬ ɪɚɡɥɢɱɧɵɦ ɢɨɧɚɦ H2Edta2–. ɋ ɞɨɛɚɜɥɟɧɢɟɦ ɚɬɨɦɚ Ɉ(6) ɤɚɠɞɵɣ ɢɡ ɚɬɨɦɨɜ ɋɚ ɷɬɨɣ ɰɟɩɨɱɤɢ ɨɤɚ- 592 ɄɊȺɌɄɂȿ ɋɈɈȻɓȿɇɂə Ɋɢɫ. 1. Ⱦɢɦɟɪ [Ca(H2O)(H2Edta)]2 ɜ ɫɬɪɭɤɬɭɪɟ I. Ɉɩɟɪɚɰɢɢ ɫɢɦɦɟɬɪɢɢ x, 0,5–y, 0,5+z; x–0,5, 0,5–y, 1–z ɢ 1,5–x, –y, 0,5+z ɞɥɹ ɚɬɨɦɨɜ O(2c), O(4c) ɢ O(8c) ɫɨɨɬɜɟɬɫɬɜɟɧɧɨ. Ⱥɬɨɦɵ ɜɨɞɨɪɨɞɚ ɧɟ ɩɨɤɚɡɚɧɵ, ɡɚ ɢɫɤɥɸɱɟɧɢɟɦ H(N). ɒɬɪɢɯɨɜɵɟ ɥɢɧɢɢ — ɜɨɞɨɪɨɞɧɵɟ ɫɜɹɡɢ N—H…O ɠɟɬɫɹ ɜ ɫɨɫɬɚɜɟ ɭɩɨɦɹɧɭɬɨɝɨ ɜɵɲɟ ɞɢɦɟɪɚ. ɉɨɫɤɨɥɶɤɭ ɞɪɭɝɢɟ ɚɬɨɦɵ ɋɚ ɞɢɦɟɪɨɜ ɬɚɤɠɟ ɜɯɨɞɹɬ ɜ ɫɨɫɬɚɜ ɞɪɭɝɢɯ ɰɟɩɨɱɟɤ, ɬɨ ɦɵ ɩɪɢɯɨɞɢɦ ɤ ɡɚɤɥɸɱɟɧɢɸ, ɱɬɨ ɫ ɭɱɚɫɬɢɟɦ ɧɚɡɜɚɧɧɵɯ ɬɪɟɯ ɚɬɨɦɨɜ ɤɢɫɥɨɪɨɞɚ ɨɞɧɨɣ ɩɨɥɨɜɢɧɵ ɢɨɧɚ H2Edta2– ɮɨɪɦɢɪɭɸɬɫɹ ɫɥɨɢ, ɩɚɪɚɥɥɟɥɶɧɵɟ ɩɥɨɫɤɨɫɬɢ xz. ɋɜɹɡɢ ɋɚ—Ɉ(2) ɢ ɋɚ—Ɉ(4) ɫ ɚɬɨɦɚɦɢ ɤɢɫɥɨɪɨɞɚ ɞɪɭɝɨɣ ɩɨɥɨɜɢɧɵ ɢɨɧɚ H2Edta2– sɫɲɢɜɚɸɬs ɫɥɨɢ ɜ ɬɪɟɯɦɟɪɧɵɣ ɤɚɪɤɚɫ (ɫɦ. ɪɢɫ. 2). ȼ ɢɨɧɟ H2Edta2–, ɤɚɤ ɨɛɵɱɧɨ, ɚɬɨɦɵ N, ɤɨɝɞɚ ɨɧɢ ɧɟ ɫɜɹɡɚɧɵ ɫ ɢɨɧɨɦ ɦɟɬɚɥɥɚ, ɨɤɚɡɵɜɚɸɬɫɹ ɩɪɨɬɨɧɢɪɨɜɚɧɧɵɦɢ ɢ ɭɱɚɫɬɜɭɸɬ ɜɨ ɜɧɭɬɪɢɦɨɥɟɤɭɥɹɪɧɵɯ ɜɨɞɨɪɨɞɧɵɯ ɫɜɹɡɹɯ [ 8, 9 ] (ɫɦ. ɪɢɫ. 1, ɬɚɛɥ. 3). ȼ ɫɬɪɭɤɬɭɪɟ I ɢɦɟɸɬɫɹ ɬɚɤɠɟ ɜɨɞɨɪɨɞɧɵɟ ɫɜɹɡɢ ɫ ɭɱɚɫɬɢɟɦ ɦɨɥɟɤɭɥɵ H2O(2w). Ʉɨɧɮɢɝɭɪɚɰɢɹ ɢɨɧɚ H2Edta2–, ɤɨɬɨɪɚɹ ɨɩɪɟɞɟɥɹɟɬɫɹ, ɜ ɱɚɫɬɧɨɫɬɢ, ɬɨɪɫɢɨɧɧɵɦ ɭɝɥɨɦ N—C—C—N ɜ ɷɬɢɥɟɧɞɢɚɦɢɧɨɜɨɦ ɮɪɚɝɦɟɧɬɟ, ɦɟɧɹɟɬɫɹ ɨɬ ɫɨɟɞɢɧɟɧɢɹ ɤ ɫɨɟɞɢɧɟɧɢɸ. Ⱦɥɹ IIa ɢ III ɷɬɨɬ ɭɝɨɥ ɪɚɜɟɧ 180° (ɪɚɡɜɟɪɧɭɬɚɹ ɮɨɪɦɚ) ɩɨ ɬɪɟɛɨɜɚɧɢɸ ɤɪɢɫɬɚɥɥɨɝɪɚɮɢɱɟɫɤɨɣ ɫɢɦɦɟɬɪɢɢ, ɚ ɞɥɹ IIb ɢ IIc — 78,6° ɢ 161,8° ɫɨɨɬɜɟɬɫɬɜɟɧɧɨ. Ⱦɥɹ ɫɬɪɭɤɬɭɪɵ I ɨɧ ɫɨɫɬɚɜɥɹɟɬ 65,4°, ɱɬɨ ɛɥɢɡɤɨ ɤ ɡɧɚɱɟɧɢɹɦ 50°—60° ɭ ɪɚɡɥɢɱɧɵɯ ɤɨɦɩɥɟɤɫɨɜ, ɝɞɟ ɢɦɟɟɬɫɹ ɷɬɢɥɟɧɞɢɚɦɢɧɨɜɵɣ ɦɟɬɚɥɥɨɰɢɤɥ. Ȼɥɚɝɨɞɚɪɹ ɬɚɤɨɣ sɫɜɟɪɧɭɬɨɣs ɮɨɪɦɟ H2Edta2– ɜ I, ɤɚɤ ɢ ɜ IIb, ɫɬɚɧɨɜɹɬɫɹ ɜɨɡɦɨɠɧɵɦɢ ɜɧɭɬɪɢɦɨɥɟɤɭɥɹɪɧɵɟ ɜɨɞɨɪɨɞɧɵɟ ɫɜɹɡɢ (ȼɋ) ɚɬɨɦɚ ɚɡɨɬɚ ɫ ɚɬɨɦɚɦɢ ɤɢɫɥɨɪɨɞɚ ɞɪɭɝɨɣ ɩɨɥɨɜɢɧɵ ɚɧɢɨɧɚ (ɩɨɡɢɰɢɢ 3 ɢ 4 ɜ ɬɚɛɥ. 3). ȼ ɤɪɢɫɬɚɥɥɟ ɫɜɨɛɨɞɧɨɣ ɤɢɫɥɨɬɵ H4Edta, ɝɞɟ ɩɪɨɬɨɧɢɪɨɜɚɧɵ ɚɬɨɦɵ ɚɡɨɬɚ ɢ ɞɜɟ ɤɚɪɛɨɤɫɢɥɶɧɵɟ ɝɪɭɩɩɵ Ɋɢɫ. 2. ɉɪɨɟɤɰɢɹ ɫɬɪɭɤɬɭɪɵ I ɜɞɨɥɶ ɨɫɢ x. Ⱥɬɨɦɵ ɜɨɞɨɪɨɞɚ ɢ O(2w) ɧɟ ɩɨɤɚɡɚɧɵ 593 ɄɊȺɌɄɂȿ ɋɈɈȻɓȿɇɂə Ɍɚɛɥɢɰɚ 3 Ƚɟɨɦɟɬɪɢɱɟɫɤɢɟ ɩɚɪɚɦɟɬɪɵ ɜɨɞɨɪɨɞɧɵɯ ɫɜɹɡɟɣ ɜ ɫɬɪɭɤɬɭɪɟ I ɋɜɹɡɶ A—H…B N(1)–H(1N)...O(1) N(1)–H(1N)...O(4) N(1)–H(1N)...O(5) N(2)–H(2N)...O(1) N(2)–H(2N)...O(5) O(1w)–H...O(2w) O(1w)–H...O(3)#1 O(1w)–H...O(3)#2 O(2w)–H...O(2)#3 O(2w)–H...O(5)#4 Ɋɚɫɫɬɨɹɧɢɟ, Å H…B A—H 0,845(16) 0,845(16) 0,845(16) 0,908(16) 0,908(16) 0,82(3) 0,82(2) 0,82(2) 0,91(2) 0,90(3) 2,303(15) 2,316(16) 2,037(16) 2,099(16) 2,309(15) 1,95(2) 2,50(2) 1,95(2) 1,94(2) 1,93(2) A…B 2,7172(14) 2,6534(14) 2,7360(14) 2,8531(14) 2,7121(14) 2,7399(17) 2,9320(17) 2,7403(18) 2,8421(16) 2,7719(15) ɍɝɨɥ AHB, ɝɪɚɞ. 110,6(12) 104,3(13) 139,7(14) 139,9(14) 106,6(11) 164(3) 114,7(18) 162(2) 171(2) 156(2) ɉ ɪ ɢ ɦ ɟ ɱ ɚ ɧ ɢ ɟ . Ɉɩɟɪɚɰɢɢ ɫɢɦɦɟɬɪɢɢ ɞɥɹ ɚɬɨɦɨɜ B: #1 x–1/2, 1/2–y, 1–z; #2 3/2–x, y–1/2, z; #3 3/2–x, –y, 1/2+z; #4 2–x, –y, 1–z. (ɤɪɢɫɬɚɥɥɨɝɪɚɮɢɱɟɫɤɚɹ ɫɢɦɦɟɬɪɢɹ 2), ɭɝɨɥ N—C—C—N ɪɚɜɟɧ 67,1° ɢ ɬɚɦ ɬɚɤɠɟ ɜɨɡɦɨɠɧɵ ɩɨɞɨɛɧɵɟ ȼɋ [ 10 ]. ɇɟɞɚɜɧɨ [ 11 ] ɨɩɭɛɥɢɤɨɜɚɧɚ ɫɬɪɭɤɬɭɪɚ ɫɨɟɞɢɧɟɧɢɹ ɢɨɧɚ ɋɚ2+ ɫ ɩɪɨɬɨɧɢɪɨɜɚɧɧɵɦ ɚɧɢɨɧɨɦ ɢɦɢɧɨɞɢɚɰɟɬɚɬɚ H2N+(CH2CO 2 )2 (Hida–) ɫɨɫɬɚɜɚ Ca(Hida)2 (IV). ɂɨɧ Hida– ɦɨɠɧɨ ɪɚɫɫɦɚɬɪɢɜɚɬɶ ɤɚɤ ɩɨɥɨɜɢɧɭ H2Edta2– ɛɟɡ ɷɬɢɥɟɧɨɜɨɝɨ ɦɨɫɬɢɤɚ, ɨɞɧɚɤɨ ɫɬɪɭɤɬɭɪɚ IV ɢɦɟɟɬ ɦɚɥɨ ɨɛɳɟɝɨ ɫ I. ɉɪɟɠɞɟ ɜɫɟɝɨ, ɨɧɚ ɫɥɨɢɫɬɚɹ, ɚɬɨɦɵ ɋɚ ɧɚɯɨɞɹɬɫɹ ɜ ɰɟɧɬɪɟ ɢɧɜɟɪɫɢɢ ɢ ɨɧɢ ɨɤɪɭɠɟɧɵ ɲɟɫɬɶɸ ɚɬɨɦɚɦɢ ɤɢɫɥɨɪɨɞɚ, ɩɪɢɧɚɞɥɟɠɚɳɢɦɢ ɲɟɫɬɢ ɫɨɫɟɞɧɢɦ ɢɨɧɚɦ Hida–, ɬɚɤ ɱɬɨ ɤɚɠɞɵɣ ɚɧɢɨɧ ɫɜɹɡɚɧ ɫ ɬɪɟɦɹ ɚɬɨɦɚɦɢ ɋɚ. ɉɪɢ ɷɬɨɦ ɢɡ ɞɜɭɯ ɚɬɨɦɨɜ ɋɚ ɢ ɞɜɭɯ ɤɚɪɛɨɤɫɢɥɶɧɵɯ ɝɪɭɩɩ ɪɚɡɥɢɱɧɵɯ ɚɧɢɨɧɨɜ ɨɛɪɚɡɭɸɬɫɹ 8-ɱɥɟɧɧɵɟ ɰɢɤɥɵ ɋɚ(ɈɋɈ)2ɋɚ, ɚ ɜ I ɰɢɤɥɵ ɛɨɥɟɟ ɩɪɨɬɹɠɟɧɧɵɟ (ɦɢɧɢɦɭɦ 16-ɱɥɟɧɧɵɟ). ɋɉɂɋɈɄ ɅɂɌȿɊȺɌɍɊɕ 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. Font-Bardia M., Solans X., Font-Altaba M. // Acta Crystallogr. – 1993. – 49C, N 8. – P. 1452 – 1456. Cotrait M. // Compt. Rend. Acad. Sci. Ser. C. – 1969. – 268, N 21. – P. 1848 – 1851. Cotrait M. // Acta Crystallogr. – 1970. – 26B, N 8. – P. 1152 – 1161. OcDonnell J.M., Day V.W., Hoard J.L. // Inorg. Chem. – 1973. – 12, N 8. – P. 1754 – 1757. Altomare A., Burla M.C., Carnalli M. et al. // J. Appl. Crystallogr. – 1999. – 32, N 1. – P. 115 – 119. Sheldrick G.M. SHELX-97. Program for the Solution and Refinement of Crystal Structures. – Germany: University of Göttingen, 1997. Spek A.L. // J. Appl. Crystallogr. – 2003. – 36, N 1. – P. 7. ɒɤɨɥɶɧɢɤɨɜɚ Ʌ.Ɇ., ɉɨɪɚɣ-Ʉɨɲɢɰ Ɇ.Ⱥ., Ⱦɹɬɥɨɜɚ ɇ.Ɇ. // ɀɭɪɧ. ɫɬɪɭɤɬɭɪ. ɯɢɦɢɢ. – 1986. – 26, ʋ 2. – ɋ. 138 – 160. ɒɤɨɥɶɧɢɤɨɜɚ Ʌ.Ɇ., ɉɨɪɚɣ-Ʉɨɲɢɰ Ɇ.Ⱥ. // ɍɫɩ. ɯɢɦɢɢ. – 1990. – 59, ʋ 7. – ɋ. 1111 – 1143. Cotrait M. // Acta Crystallogr. – 1972. – 28B, N 3. – P. 781 – 785. ɉɨɥɹɤɨɜɚ ɂ.ɇ., ɉɨɡɧɹɤ Ⱥ.Ʌ., ɋɟɪɝɢɟɧɤɨ ȼ.ɋ. // ɀɭɪɧ. ɧɟɨɪɝɚɧ. ɯɢɦɢɢ. – 2003. – 48, ʋ 12. – ɋ. 1998 – 2003.